Abstract
INTRODUCTION: Most anemic non-del(5q) lower risk myelodyplastic syndromes (MDS) patients are treated with erythropoiesis stimulating agents (ESAs). Second-line treatments, including hypomethylating agents (HMA), lenalidomide (LEN) and other drugs, may be used after ESA failure in some countries, but their effect on disease progression, transformation to acute myeloid leukemia (AML), and overall survival (OS) is unknown. We analyzed MDS patients' outcome after primary or secondary failure of treatment with ESAs as a first-line treatment option.
METHODS: This retrospective study included 1065 non-del(5q) lower risk (low or intermediate-1 IPSS risk) MDS patients, registered in the Hellenic National MDS Registry, treated with ESAs. Extensive biostatistical analysis performed in this study included Kaplan-Meier survival analysis. The level of statistical significance was set at a probability value of less than 0.050 (P <0.050).
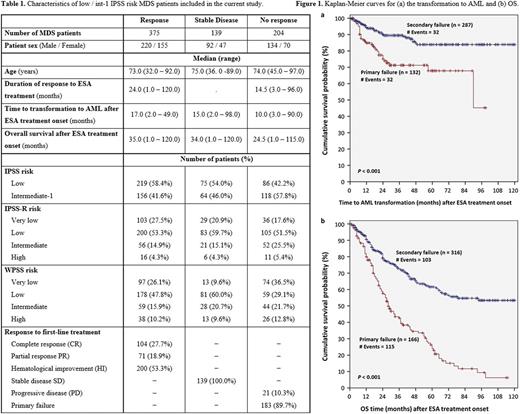
RESULTS: Baseline patient characteristics are presented in Table 1. 848 (79.6%) of 1065 MDS patients included in this study were first treated with ESAs. Erythroid response (HI-E) to ESAs was 26.3%, and the median response duration was 21 months. Stable disease (SD) was achieved by 139 (19.4%) MDS patients, while 204 (28.4%) patients receiving ESA had progression of disease (PD) or primary treatment failure. Moreover, 353 (41.6%) MDS patients relapsed after an initial response. Primary failure to ESA was associated with a higher risk of transformation to AML, as well as poorer prognostic outcome regarding OS. The 5-year cumulative incidence of AML transformation in patients with primary and secondary failure was 32.2% and 16.1% respectively (P <0.001). In a median follow-up time period of 28 months, the mean survival from ESA failure was 40 and 80 months for primary refractory patients and relapsing patients, respectively (P <0.001). The estimated mean time to transformation to AML patients with primary failure to ESA treatment was 71.2 months (95% confidence interval [CI] = 63.0-79.3) vs. 104.6 months (95% CI = 99.6-109.6) for those having a secondary failure (P <0.001) (Figure 1a). The estimated mean OS for patients with primary failure to ESA treatment was 40.1 months (95% CI = 34.5 - 45.6) vs. 79.9 months (95% CI = 73.9-85.9) for those having a secondary failure (P <0.001) (Figure 1b). Out of 412 patients who received a second-line treatment, 48 received HMAs, 22 were treated with LEN and 176 had other treatments.
CONCLUSIONS: The data of this large, multicenter, retrospective cohort of non-del(5q) lower-risk MDS patients, treated with ESAs, show that patients not responding to ESAs, as first-line treatment, are at a higher risk of transformation to AML and have poorer OS, compared to those who initially respond to ESAs but lose response thereafter. This information combined with other parameters, predictive a worse outcome (eg IPSS-R, duration of ESAs response) can be used in future clinical studies, distinguishing candidate patients for a more intensive treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal